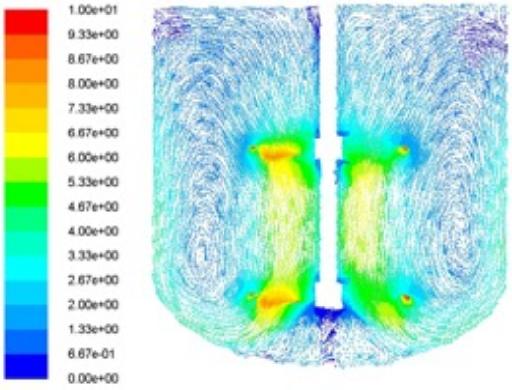
LOTUS Mixers has extensive experience in cGMP facilities for both upstream and downstream pharmaceutical processes.
Vaccine Production
Antibiotics
Mammalian cell cultures
Blood factors injectables
Buffer solutions
API production
LOTUS MIXERS offers our customers the support and knowledge to help in scale up, process development (in terms of mixing performance), and design for cGMP installations/compliance.
Our background in the production allows us to differentiate between mixing theory and practice for successful operation in manufacturing operations.
Proper mixing is critical to both homogeneous and heterogeneous reactions either in achieving the desired conversion rate and/or in achieving the predicted selectivity.
Another critical application is the crystallization processes which requires extensive knowledge in mixing in order to realize the desired crystal attributes on scale-up.
LOTUS Mixers - Pharmaceutical Applications
LOTUS MIXERS Inc.
3449 Technology Drive # 201
North Venice, FL 34275 USA
Anytime, you have a question about mixing, agitator technology or the application for a mixer, please call (941)966-1885, email (info@lotusmixers.com) or use our on-line data sheet.

An American Agitator Company
Better Designs -Best Total Cost of Ownership
Top Entry - SIde Entry - Bottom Entry -Magnetic Drive
Static and Portable Mixers